The FDA has issued a recall for a boutique hand sanitizer brand, citing potential blindness and coma as harmful side effects.
40 batches of Aruba Aloe Balm, Aruba Aloe hand Sanitizer Gel Alcohol 80%, and Aruba Aloe Alcoholada Gel have been recalled.
Methanol, a toxic agent found in vehicle antifreeze liquid, was discovered in the tainted batches of hand sanitizer.
Common side effects of the toxic substance include the usual vomiting, nausea, and headaches, which are common to almost all illnesses.
However, included in the long list were the far more serious side effects of coma, blindness, seizures, and even death.
Make sure to read on and stop using these products immediately if they are in your household—throw them away.
Aruba Aloe Balm N.V. Issues Voluntary Nationwide Recall of Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholada Gel Due to Presence of Methanol https://t.co/QgqraeEXyr pic.twitter.com/nfEvzBOWGY
— U.S. FDA Recalls (@FDArecalls) April 8, 2024
Aruba Aloe Hand Sanitizer Gel Alcohol 80% and Aruba Aloe Alcoholada Gel are the culprits.
METHANOL is the ingredient, a toxic alcohol found in antifreeze and windshield washer fluid. https://t.co/2kGh1nZjkx pic.twitter.com/fnzN5DMrdt— JohnnyNoble💧 (@JohnnyNoblebody) April 9, 2024
The Epoch Times explained:
The firm did not say in the notice how its products were contaminated with methanol or how the error was found.
Methanol, known as wood alcohol, is described by federal officials as “extremely poisonous” and that as little as two tablespoons “can be deadly to a child,” according to the MedcinePlus website.
The Food and Drug Administration provided these tables of tainted product batches organized by lot number and expiration date.

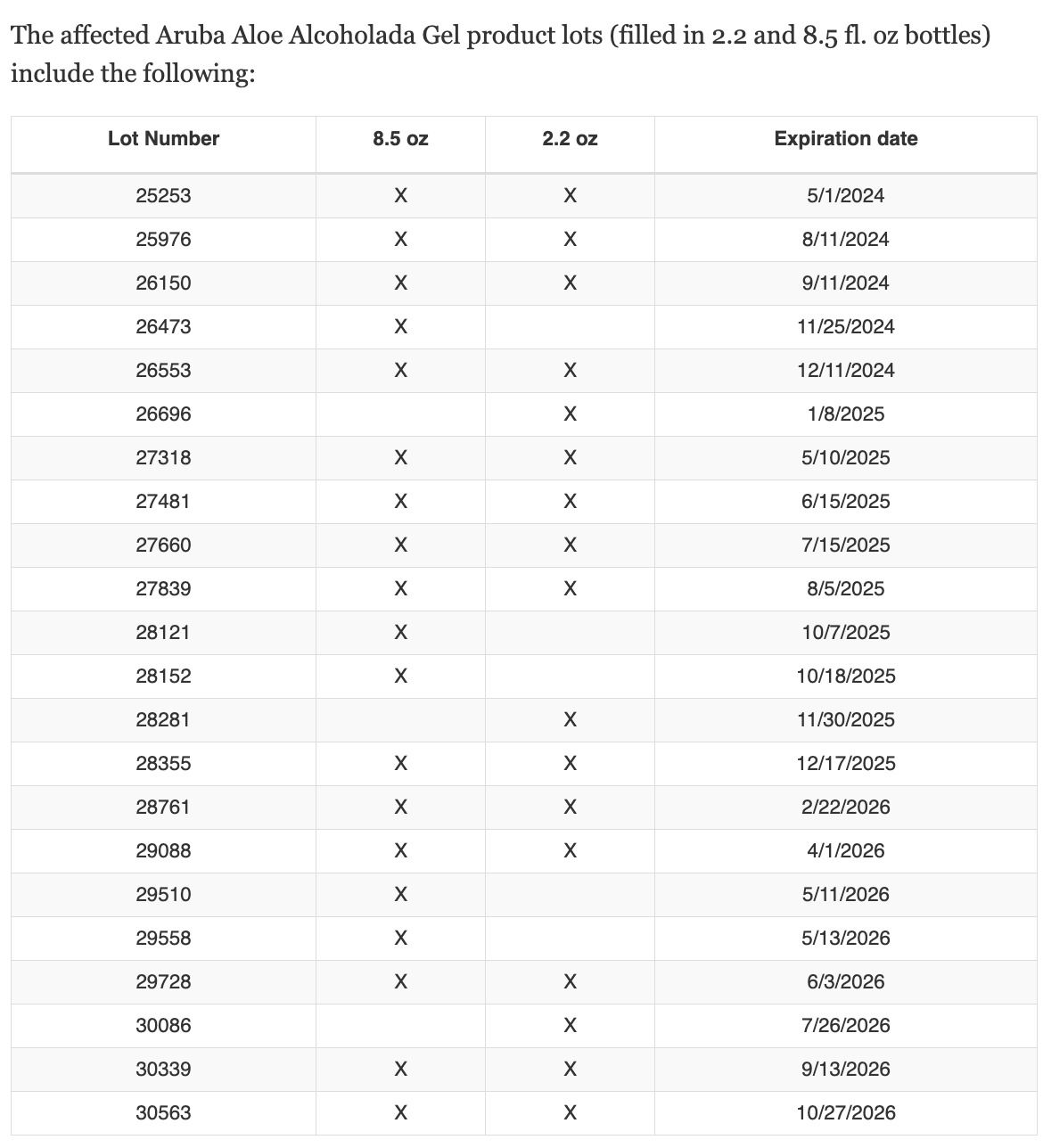
CBS News added:
The recalled products were distributed between May 1, 2021, and Oct. 27, 2023, and sold in the U.S. online through the Aruba Aloe Balm website.
The company is notifying consumers who purchased the products by email and offering a discount coupon for a next purchase.
Those who purchased the recalled products should discard them.


Join the conversation!
Please share your thoughts about this article below. We value your opinions, and would love to see you add to the discussion!